R & D pipelines
Acrom line-up
DIRECTION
- 1. It is determined as a comprehensive score of future expected insight, business feasibility and marketability in this year
- 2. Materials and phrases available with maximum, Best, and first maximization - individual approved HFFI projects
- 3. Consideration of Korea's new market opening, high scalability, new formulation, convenience, and price factors
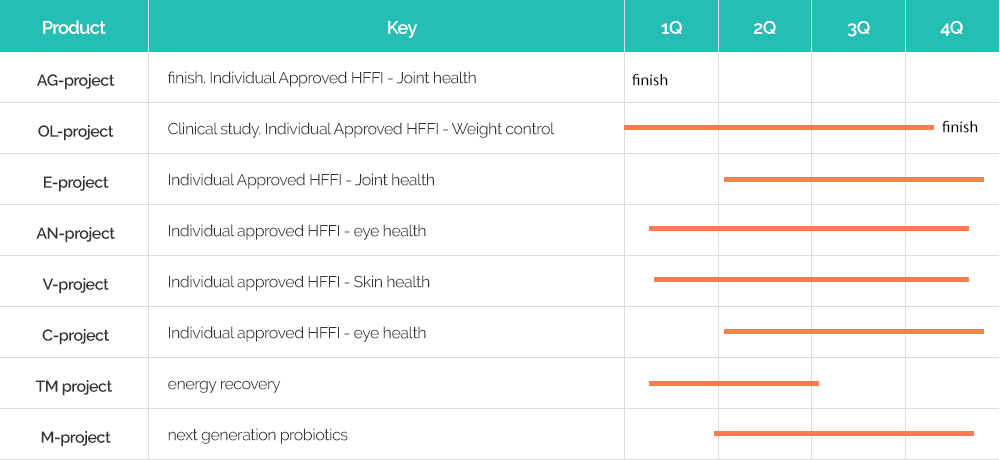 ※ Please understand that some projects are carried out under the NDA contract with the company, so we cannot clearly mark them.
※ Please understand that some projects are carried out under the NDA contract with the company, so we cannot clearly mark them.Korea MFDS System
Classification of health functional foods
The current regulation
Notified HFFI to be listed
Ministry of Food and Drug Safety has set specifications for 68 ingredients.Manufacturing standards, test methods, functional contents, daily intake, specifications, etc.
Individually approved HFFI
A system in which a company obtains licensing from the Ministry of Food and Drug Safety by proving the standardization, non-clinical, clinical, testing methods, functional contents, safety, etc. of new materials other than the notification type.
- 1. A system that can be imported and distributed regardless of any company as long as it conforms to the standard.
- 2. It's hard to differentiate to buy in market.
- 3. Price competition intensifies (red ocean)
- 1. The system that can be imported and distributed by the only licensed companies. It becomes illegal for an unlicensed company to import and distribute
- 2. It is easy to differentiate in the market in terms of distributor.
- 3. Out of the price competition, price creation is possible. ( blue ocean)
If new materials or unique ingredients are approved for individual approved HFFI,
they can be differentiated and distributed with exclusive status. (Duration required 1 to 3 years)
they can be differentiated and distributed with exclusive status. (Duration required 1 to 3 years)
